In fluence of yttrium on microstructure and properties of Ni-Al alloy coatings prepared by laser cladding
Cun-shan WANG
State Key Lab for Materials Modi fi cation,Dalian University of Technology,Dalian 116024,China
1.Introduction
In recent years,the ordered B2-Ni-Al intermetallic compound has attracted considerable attention owing to its high melting temperature,good oxidation resistance,high thermal conductivity,and low density for potential high temperature structural application[1-3].Therefore,many researchers have tried to use the intermetallic compound as protective coating material,and the adopted techniques mainly include thermal spraying,magnetron sputtering,electron-beam physical vapor deposition,hard facing,and laser cladding,etc[4-10].Amongst these,laser cladding,as an advanced surface modifi cation technique,has been used to prepare the coatings with high density and almost zero porosity content[11].
In the present study,the Ni-Al alloy coatings with different Y additions were prepared on the 45#medium steel(containing about 0.45 wt.%C)by laser cladding,and the in fluences of Y contents on the microstructure and properties of the Ni-Al alloy coatings were systematically investigated.
2.Experimental procedure
45#medium steel with dimension of 20 mm×10 mm×10 mm is chosen as a substrate material.The powders of Ni(99.99%purity,-200 mesh),Al(99.90%purity,-200 mesh),and Y(99.99%purity,-200 mesh)are blended using ball milling according to the chemical compositions of cladding powders listed in Table 1.The powders are placed on the surface of the 45#steel to form a 1 mm thick layer,and are melted using a 5 kW continuous wave CO2laser beam.The laser cladding is performed with 3 mm diameter laser beam at a laser power of 3.8 kW and a scanning velocity of 4 mm/s.Argon gas is introduced into a melt pool to prevent the penetration of exterior oxygen during the laser cladding process.
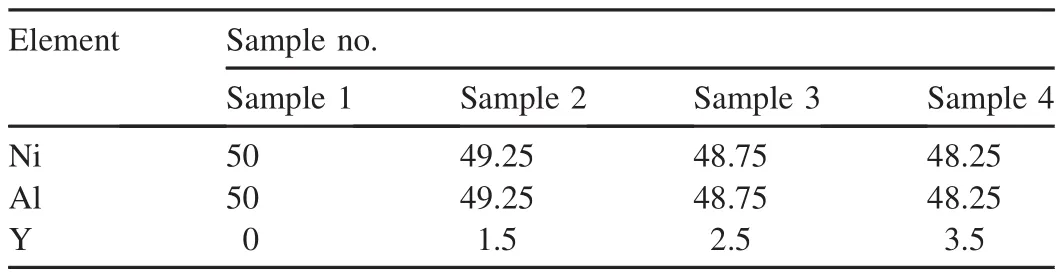
Table 1 Chemical compositions of cladding powders(at.%).
Phase identi fi cation of these coatings is carried out using Cu Kα radiation by means of XRD-6000 X-ray diffractometer.The microstructure characteristics and compositions of coatings are analyzed using JEOL-5600LV scanning electron microscopy (SEM) and EPMA-1720 electron probe microanalyzer(EPMA).The Vickers hardness of coating is measured with DMH-2LS micro-hardness tester under a load of 0.981 N and a dwell time of 15 s.The ball-and-disk friction and wear test of the cladding layers is carried out on CETR UMT-2 testing machine.A bearing steel ball(GCr15)with a diameter of 5 mm and a hardness of 55HRC is selected as wear couple.The experiment is performed with a normal load of 5 N,a sliding speed of 2 mm/s,and a wear time of 30 min.Continuousvariable-temperatureoxidation experimentis performed on SDT-Q600 thermal analyzer at a heating rate of 1.33 K/s and a temperature range of 150-1000°C.The specimens(3 mm×2.5 mm×0.4 mm)for test are cut from the laser cladding layers.
3.Results and discussion
3.1.Microstructure
Fig.1 shows an X-ray diffraction pattern taken from the Ni-Al cladding layer.Only Ni-Al intermetallic compound with the ordered cubic B2 crystal structure is indenti fi ed from the diffraction pattern,indicating that the cladding layer mainly consists of NiAl phase.
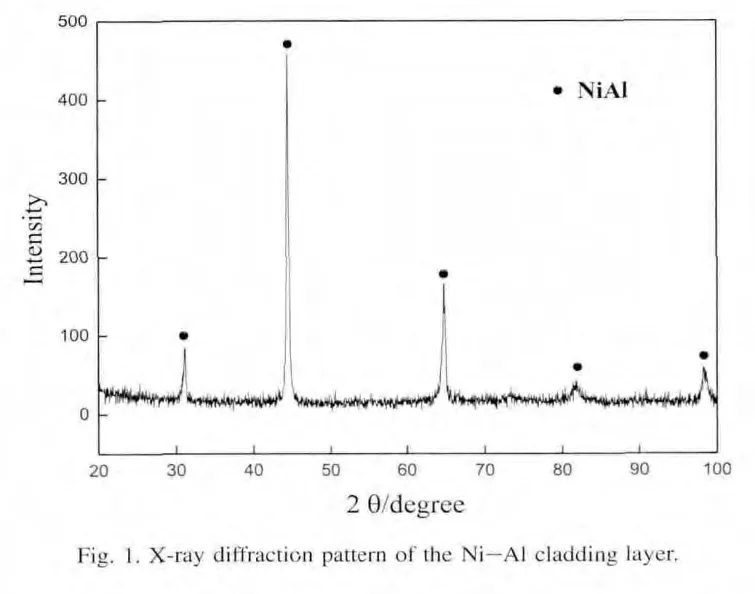
Fig.2 shows a cross-sectional SEM micrograph taken from the cladding layer.It can be found that the microstructure of the cladding layer is featured with dendrites(Fig.2(a)).Further,EPMA analysis reveals that the NiAl dendrite contains a small amount of Fe besides Ni and Al,and its composition is Ni41.78Al53.75Fe4.47.This indicates that the cladding layer is diluted by the substrate during laser cladding.In addition,oxygen element distributed between dendrites is also found in the cladding layer.From the EPMA area analysis shown in Fig.3,it can be deduced that O may react with Al to form Al2O3due to the higher binding energy between O and Al.The interface region between the cladding layer and the substrate is located at a region in which the substrate material is re-melted and reheated and new cladding material begins to be added.This makes the microstructure of the interface region totally different from that of the cladding layer,as shown in Fig.2(b).High dilution and low cooling rate result in the formation of fl at crystal with an average thickness of 21.7 μm,and the composition of the fl at crystal measured by EPMA is Ni39.51Al45.25Fe15.24.Above the interface,because the heat is flowing out of the cladding layer towards the substrate,the dendrites are more columnar and perpendicular to the interface.In the heat-affected of the substrate,because austenitic transformation takes place at higher temperature,on a subsequent cooling,a martensitic structure is formed,as shown in Fig.2(c).
Fig.4 shows the X-ray diffraction patterns of cladding layers with different Y additions.When the atomic percent of Yaddition is 1.5%,the phase constituents of the cladding layer remain unchanged,which still consist of NiAl phase.However,when the atomic percent of Y addition exceeds 1.5%limit,the phase constituents of the cladding layers change obviously.Besides the NiAl phase,a Y2O3phase in the cladding layer with 2.5 at.%Y addition and an Al5Y3O12phase in the cladding layer with 3.5 at.%Y addition are observed.
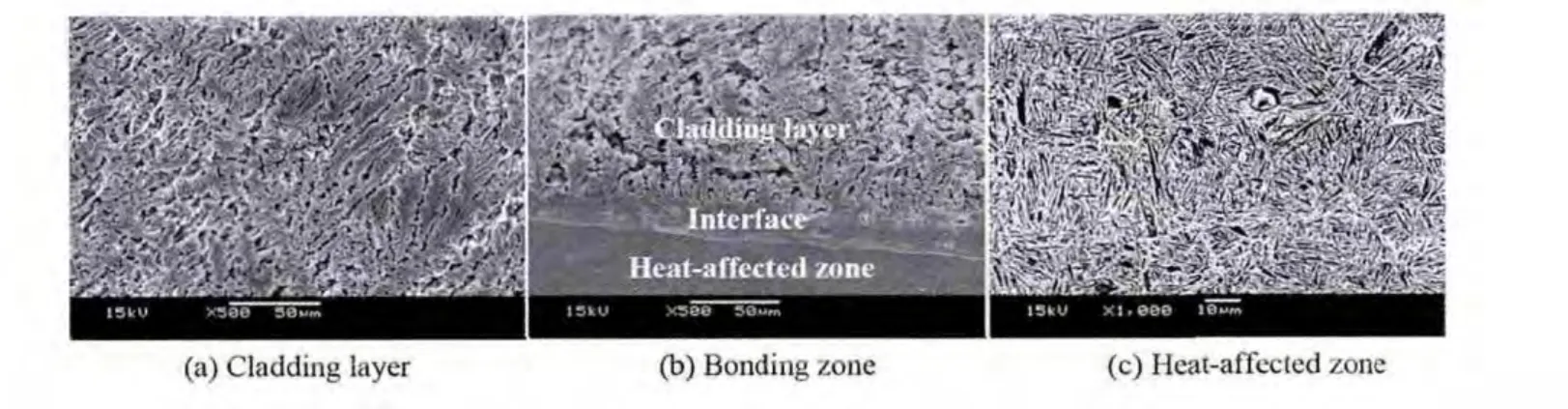
Fig.2.Cross-sectional SEM micrographs of the Ni-Al cladding layer.
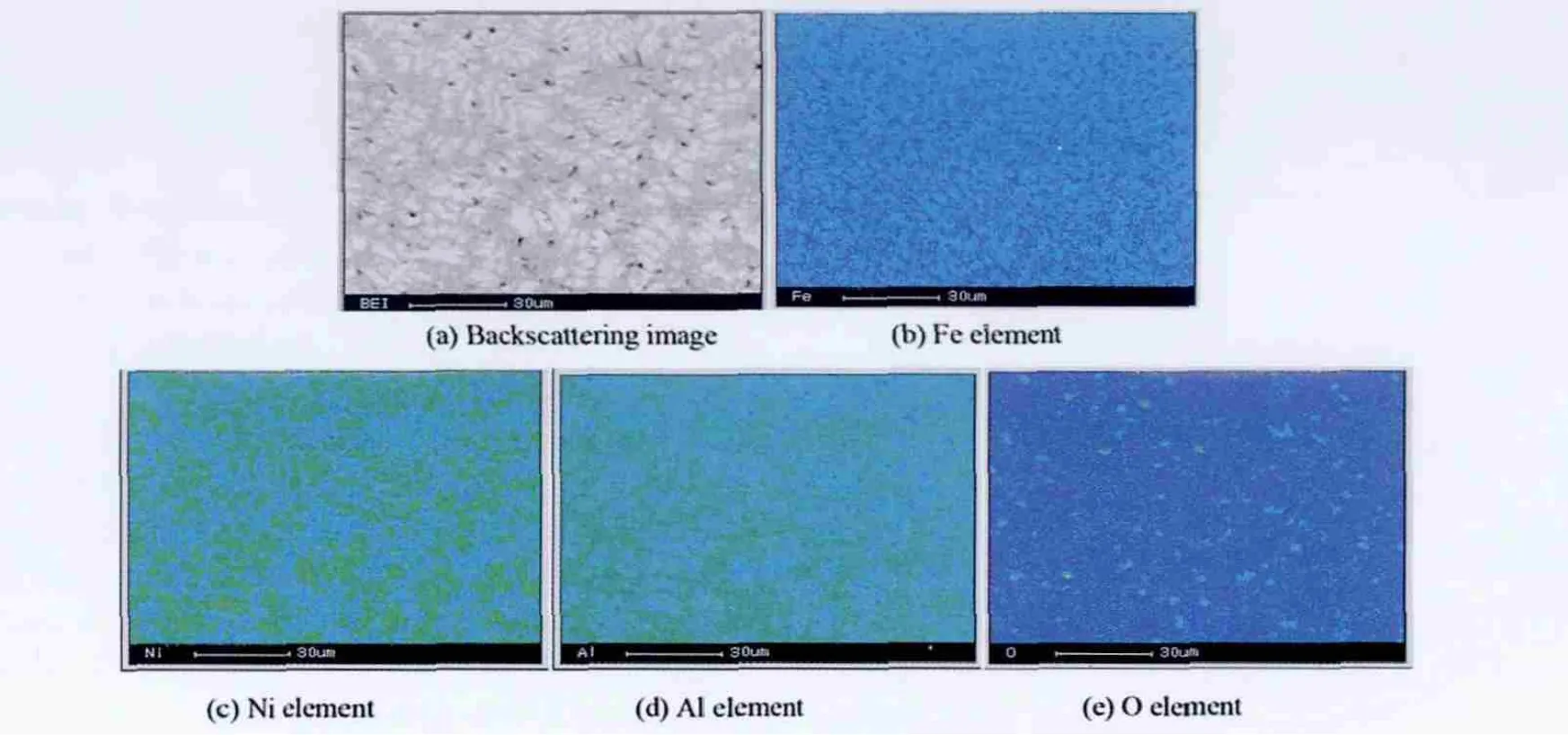
Fig.3.Area distributions of the elements in the Ni-Al cladding layer.

Fig.4.X-ray diffraction patterns of the cladding layers with different Y additions.
Fig.5 shows the typical SEM micrographs of the Ni-Al cladding layers with different Yadditions.It can be found that all cladding layers have the morphological characteristics of dendrites,but the sizes of the primary dendrites are decreased and the secondary dendrites are more pronounced with the increase in Y additions.Further,the EMPA area analysis reveals that no oxygen element is found in the cladding layer with 1.5 at.%Y addition,indicating that Y effectively puri fi es the Ni-Al alloy.However,when the atomic percent of Y addition exceeds 1.5%,O elements enriched at the tops of the cladding layers are observed.As with XRD analysis,the extra Y addition will react with O to form Y2O3oxide at higher temperature,even to form Al5Y3O12oxide as Y additions are further increased to 3.5 at.%.On a subsequent cooling process,because of high cooling rate,it is dif fi cult for those oxides to fully over flow from the molten pool,some of them are finally preserved.Those oxides enriched at the tops of the cladding layers behave as a slag former,which will be bene fi cial to inhibiting the penetration of exterior oxygen.In addition,because the Y purifying action is bene fi cial to improve the under-cooling degree of liquid phase,dendrites can be gradually re fined by increasing the under-cooling degree with Y additions.
3.2.Micro-hardness

Fig.5.SEM micrographs of the Ni-Al cladding layers with different Y additions.
Fig.6 shows the micro-hardness plots along the crosssection of the coatings with different Y additions.There are four plateaus corresponding to the four zones mentionedabove in the micro-hardness plots.The hardness value decreases in the following order:cladding layer-bonding zone-heat-affected zone-substrate.This trend can be understood according to the microstructure in each zone.In addition,it can be seen from Fig.6 that the Y addition reduces the hardness value of the Ni-Al cladding layer due to its inhibiting effect on the formation of the dispersed Al2O3oxide.However,when the atomic percent of Y addition exceeds 1.5%,the decreasing trend is lightened gradually due to re finement of grain and the enhancement of Y2O3and Al5Y3O12oxides distributed at the top of the cladding layers.
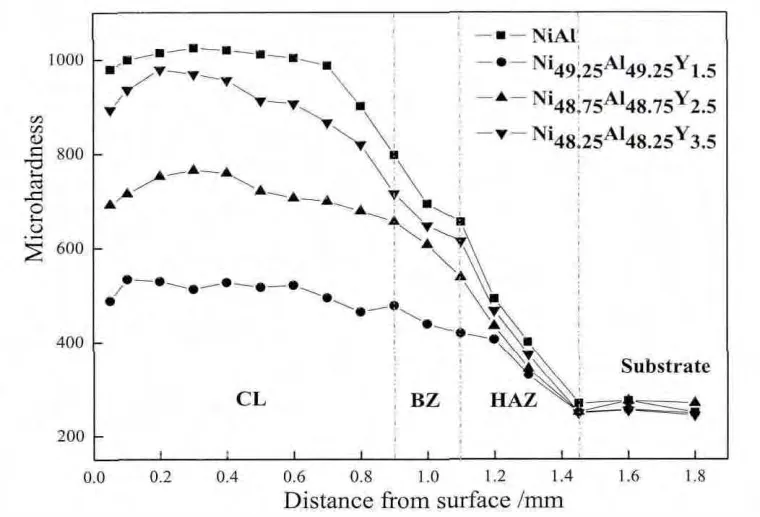
Fig.6.Micro-hardness plots along the cross-sections of the Ni-Al alloy coatings with different Y additions.
3.3.Tribological properties
Fig.7 shows the in fluence of the Y contents on the friction coefficients of the cladding layers.It can be seen that the Y addition increases the friction coefficients of the cladding layers,but the increasing degree decreases gradually with the increase in Yadditions.Despite of the high friction coefficient,the cladding layers with Y additions exhibit signi fi cantly improved wear resistances in comparison with the cladding layer without Yaddition.As shown in Fig.8(a),since some of Al2O3particles are scaled off under the alternating stress,and then join the wear process as wear materials,a signi fi cant abrasive wear with the furrow feature occurs on the worn surface of the cladding layer without Y addition.When 1.5 at.%Y addition is added to the cladding layer,the wear mechanism changes obviously because the adhesiveness between the cladding layer and the wear couple increases with the disappearance of the dispersed Al2O3phase,and only tearing pit induced by adhesive wear is observed(Fig.8(b)).However,when the atomic percent of Y addition exceeds 1.5%,because the Y2O3oxide is distributed at the top of the cladding layer with 2.5 at.%Y addition,and the Al5Y3O12oxide is distributed at the top of the cladding layer with 3.5 at.%Y addition,the adhesive wear and abrasive wear mechanisms play their respective roles on the both cladding layers simultaneously,and the tendency of the abrasive wear is heightened with the increase in Y additions.However,compared to the cladding layer without Yaddition,the furrows on the worn surfaces of the cladding layers with 2.5 at.%and 3.5 at.%Yadditions are narrow in width and shallow in depth(Fig.8(c)and(d)).The wear volumes of 0,1.5,2.5,and 3.5 at.%Y additions are 1.20×10-3,0.76×10-3,0.81×10-3,and 0.90×10-3mm3,respectively(Fig.9).This suggests that the cladding layer with 1.5 at.%Y addition has the highest wear resistance.

Fig.7.In fluence of Y contents on friction coefficients of the Ni-Al cladding layers.
3.4.Oxidation behavior
Fig.10 shows the continuous variable-temperature oxidation kinetic curves of the cladding layers with different Y additions.During continuous heating-up process,those cladding layers experience two stages:stable oxidation and severe oxidation.At the stable oxidation stage,the messes of the cladding layers are approximately unchanged with temperature as a result of the formation of a surface α-Al2O3scale which behaves as a protective layer.However,when the oxidation process begins to enter the severe oxidation stage,the messes of the cladding layers increase sharply with the increase in temperature,but the increasing trend is governed by different Y additions.As shown in Fig.10,the mass gains of the cladding layers decrease sharply with the increase in Y additions up to 1.5 vol.%,and then start to increase with the further increase in Yadditions,i.e.,the cladding layer with 1.5 at.%Y addition has the lowest mass gain,while the cladding layer without Y addition has the highest mass gain.
In order to understand the changes,the oxidation surface morphologies of the cladding layers are observed by SEM.As shown in Fig.11(a),only minor scale spallations occur on the surface of the cladding layer with 1.5 at.%Y addition.However,when the atomic percent of Y addition exceeds 1.5%,signi fi cant scale spallations are observed,and the spalled areas increase gradually with the increase of Yadditions(Fig.11(b)and(c)).As for the cladding layer without Y addition,the spalled areas further increase(Fig.11(d)).This indicates that the existences of Al2O3,Y2O3,and Al5Y3O12oxides have great effects on the adhesion of the scale.Further,XRD analysis reveals that part of the Ni-Al phases exposed by spallation transform into Ni3Al phase due to Al loss,but the cladding layer with 1.5 at.%Y addition is an exception,only Al2O3and NiAl phases are found on the oxidation surface,because the protective scale is relatively compact and full.

Fig.8.Worn surface micrographs of the Ni-Al cladding layers with different Y additions.
4.Conclusions
1)The Ni-Al cladding layers with different Y additions are mainly composed of NiAl dendrites,but what changes is that the dendrites is gradually fine with the increase of Y additions.The atomic percent of Yaddition up to 1.5%can effectively prevent Al2O3oxide from forming.However,when the atomic percent of Y addition exceeds the 1.5%limit,the extra Y addition will react with O to form Y2O3oxide,even to form Al5Y3O12oxide,depending on the amount of Y added.
2)The Y addition in a range of 1.5-3.5 at.%reduces the hardness and anti-attrition of Ni-Al cladding layer,but improves signi fi cantly its wear and oxidation resistances.
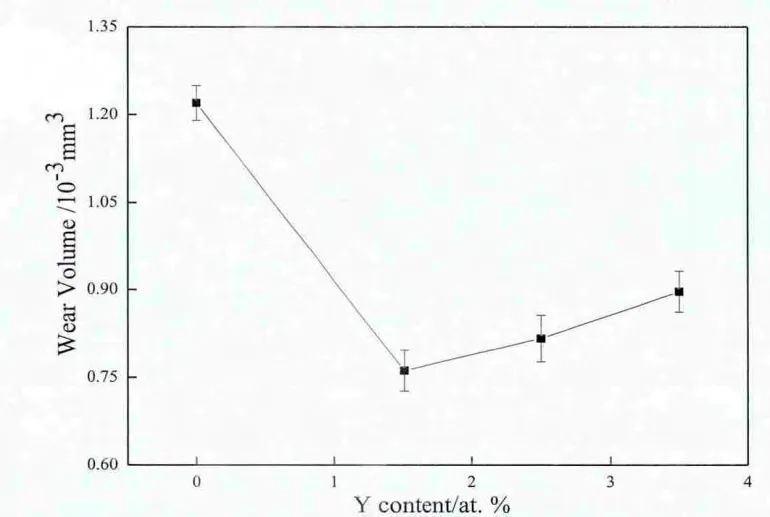
Fig.9.Wear volumes of the Ni-Al cladding layers with different Yadditions.
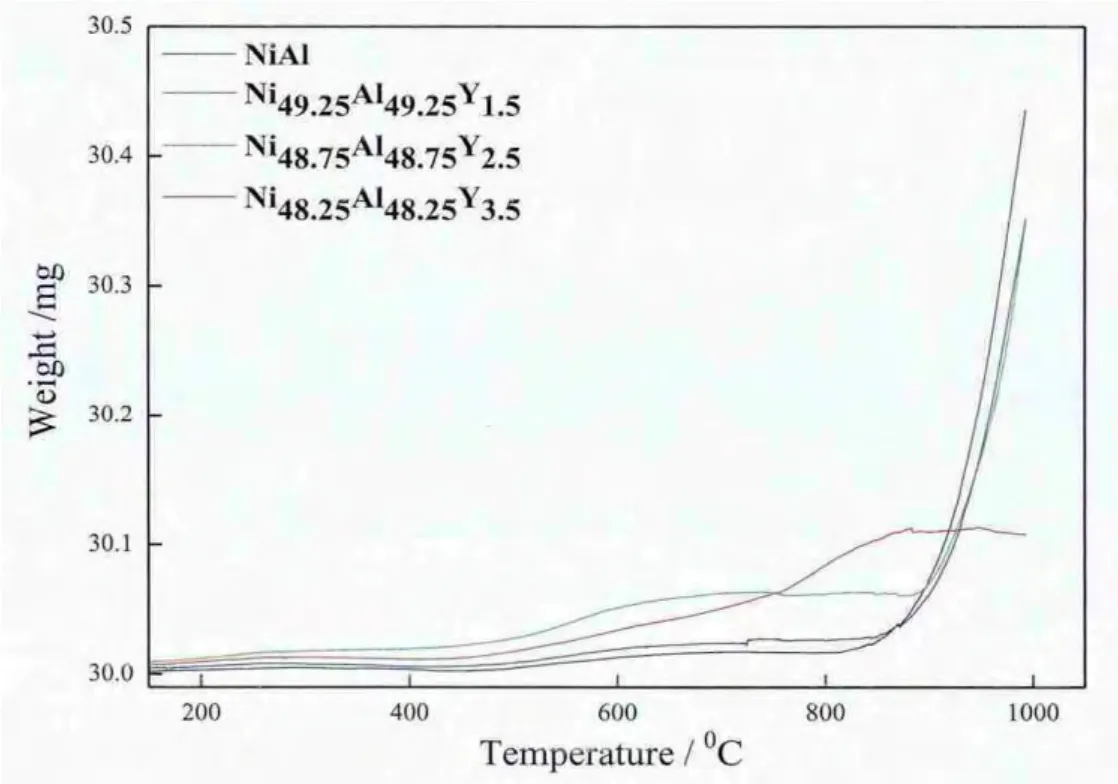
Fig.10.Continuous variable-temperature oxidation kinetic curves of the Ni-Al cladding layers with different Y additions.
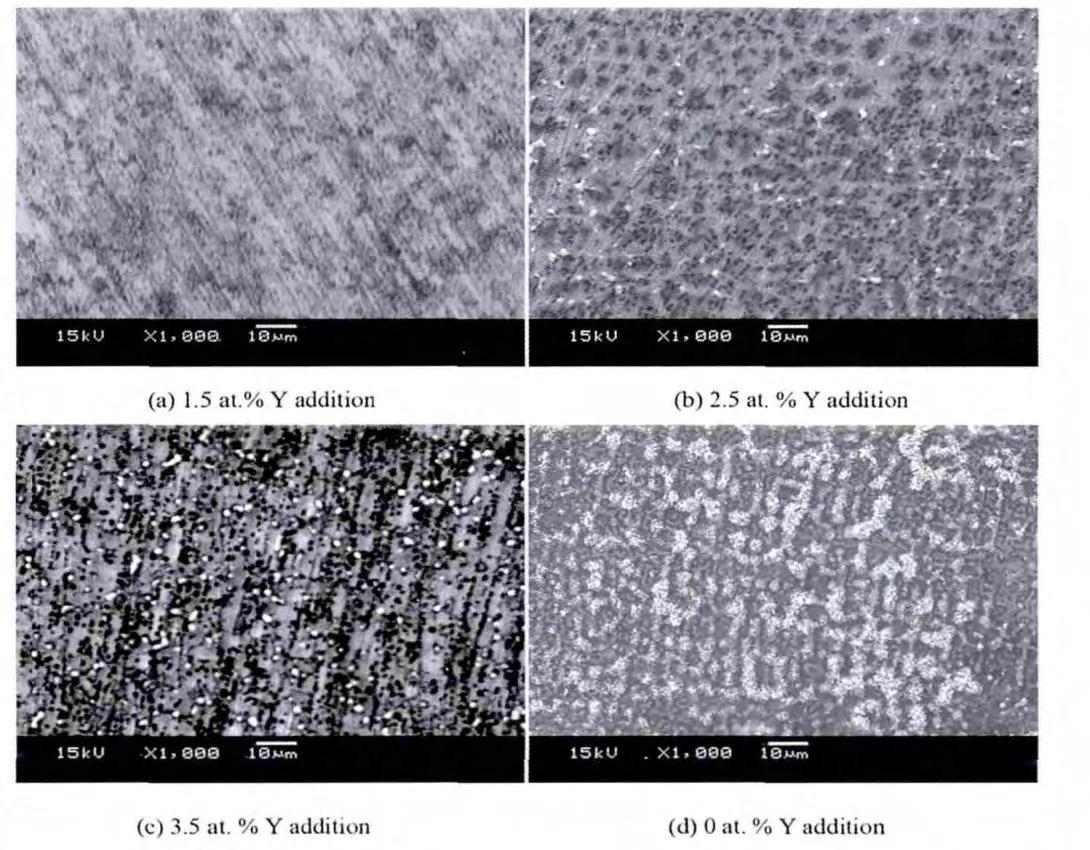
Fig.11.The oxidation surface morphologies of the Ni-Al cladding layers with different Y additions.
[1]Czeppe T,Wierzbinski S.Int J Mech Sci 2000;42(8):1499-518.
[2]Enayati MH,Karimazadeh F,Anvari SZ.J Mater Process Technol 2008;200(1):312-5.
[3]Yang SL,Wang FH,Wu WT.Intermetallics 2001;9(8):741-4.
[4]Wang Y,Wang Z,Yang Y,Chen W.Intermetallics 2008;16(5):682-8.
[5]Hearley JA, Little JA, Sturgeon AJ. Surf Coating Technol 2000;123(2):210-8.
[6]Zhong D,Moore JJ,Sutter E,Mishra B.Surf Coating Technol 2005;200(5):1236-41.
[7]Chang JT,Davison A,He JL,Matthews A.Surf Coating Technol 2006;200(20):5877-83.
[8]Yang SL, Wang FH, Sun ZM, Zhu SL. Intermetallics 2002;10(5):467-71.
[9]Li HF,Sun LD,Hesnawi A,Zhou ZH,Gong SK.Surf Coating Technol 2007;201(9):5161-4.
[10]Guo HB,Sun LD,LiHF,Gong SK.Thin Solid Films 2008;516(16):5732-5.
[11]Partes K,Giolli C,Borgioli F,Bardi U,Seefeld T,Vollertsen F.Surf Coating Technol 2008;202(10):2208-13.
- Defence Technology的其它文章
- Estimation of the kinetic parameters for thermal decomposition of HNIW and its adiabatic time-to-explosion by Kooij formula
- Effect of welding processes and consumables on fatigue crack growth behaviour of armour grade quenched and tempered steel joints
- Research on design and firing performance of Si-based detonator
- Analysis of hydrodynamic characteristics of unmanned underwater vehicle moving close to the sea bottom
- Dynamic globularization prediction during cogging process of large size TC11 titanium alloy billet with lamellar structure
- A numerical study on the disturbance of explosive reactive armors to jet penetration

